New article by Tara Callinan 'How AI, Machine Learning and Automation will Impact Business in 2018 and Beyond' gives an overview of what technology trends to watch for and how they will impact businesses in the near future.
Marketing Gets Smarter with AI and Machine Learning
What to expect?
- Dynamic landing pages and websites.
- More personalized ads.
- Nurturing leads through social media through personalized, real-time content targeting.
E-Commerce Reaches New Heights
Machine learning analyzes data based on a user’s purchase history or online shopping behavior. This helps to further personalize purchasing experience. And when it’s time to purchase, machine learning is applied to reduce the risk of credit fraud in small businesses.
Integrating Chatbots
Chatbots will play a key role in the future of customer service:
- Giving 24/7 customer service
- The era of being ‘on hold’ is gone
- Quick access to customer data makes service more personal
Automation Now and in 2018
Though Machine Learning and AI are hot topics in the tech world, it is not to a point that small to medium size businesses can leverage it in the immediate future. But there is still hope for them with automation. Powered by the Cloud, this type of technology has already revolutionized Marketing and Sales workflows and interactions but it is also starting to touch the various other parts of a business.
For example automation of:
- operations
- accounting
- payroll/HR.
Find more information on Tenfold Marketing Blog.
Friday, December 22, 2017
How new technologies will Impact Business in the upcoming 2018
Monday, November 27, 2017
ISO 9001:2015 and Business management system
Can we assume that ISO 9001-2015 can be used as a Business management system?
In which case that can be possible?
ISO 9001:2015, like the earlier versions of the Standard, was created precisely for this purpose.
We just have to take into account that it is very difficult to develop common requirements for the management of completely different organizations: large and small, focused on production and services, successful and not so much, with different production orientation, with different ownership type, etc..
Therefore, ISO 9001:2015 provides the minimum set of universal requirements that are used as a basis.
Further, each organization builds its Business management system, taking into account the peculiarities of their own business. Common areas of this development are the application of such methodologies as TQM, Lean-Six Sigma.
In which case that can be possible?
ISO 9001:2015, like the earlier versions of the Standard, was created precisely for this purpose.
We just have to take into account that it is very difficult to develop common requirements for the management of completely different organizations: large and small, focused on production and services, successful and not so much, with different production orientation, with different ownership type, etc..
Therefore, ISO 9001:2015 provides the minimum set of universal requirements that are used as a basis.
Further, each organization builds its Business management system, taking into account the peculiarities of their own business. Common areas of this development are the application of such methodologies as TQM, Lean-Six Sigma.
Monday, November 20, 2017
ISO 45001 Audit Checklist
Introducing new product - ISO 45001 Audit checklist.
42 pages editable MS Word document with detailed explanations, auditor tips and recommendations - our ISO 45001 Audit checklist can be utilized in a number of ways. The ISO 45001:2018 Audit checklist:
ISO 45001:2018 Audit Checklist completely covers ISO 45001:2018 conformance and performance audits.
Download a .PDF preview of the ISO 45001:2018 Audit Checklist.
More information and the latest pricing updates on our site.
42 pages editable MS Word document with detailed explanations, auditor tips and recommendations - our ISO 45001 Audit checklist can be utilized in a number of ways. The ISO 45001:2018 Audit checklist:
- provides a complete understanding of how to verify compliance with the requirements of all ISO 45001:2018 clauses;
- can be used as practice for internal auditors workshops.
- allows every employee to check his/her activity performance in compliance with the new requirements of ISO 45001:2018.
ISO 45001:2018 Audit Checklist completely covers ISO 45001:2018 conformance and performance audits.
Download a .PDF preview of the ISO 45001:2018 Audit Checklist.
More information and the latest pricing updates on our site.
Monday, October 30, 2017
ISO 45001:2018 Transition Gap Analysis
In anticipation of the ISO 45001:2018 updates, we introduce new product - ISO 45001:2018 Transition Gap Analysis with the guidelines for transition.
The comparison materials are presented in a table format, containing ISO 45001:2018 Clauses, corresponding OHSAS 18001:2007 and ISO 9001:2015 clauses, content of changes and recommended actions to transfer the OH&S to the new version (if changes are present).
The product features
The comparison materials are presented in a table format, containing ISO 45001:2018 Clauses, corresponding OHSAS 18001:2007 and ISO 9001:2015 clauses, content of changes and recommended actions to transfer the OH&S to the new version (if changes are present).
The product features
- Traditional clauses conformity table of OHSAS 18001:2007 and New versions of ISO 45001:2018 and ISO 9001:2015.
- Analysis of changes.
- Implementation recommendations for each change.
- Easy to use table format
- Printable PDF
- 28 pages
For a limited in October/November 2017 we offer this document as a free download. Visit our website to claim your copy.
Labels:
iso 45001 2018 transition,
iso 45001 2018 updates,
occupational safety management system,
ohs system gap analysis
Friday, October 27, 2017
ISO 9001/14001/45001 Requirements Comparison
The most recent versions of ISO Management Standards (ISO 9001:2015, ISO 14001:2015, ISO 45001:2018) have identical structure.
This can be very useful when building an Integrated Management System taking into account several standards requirements.
All three international standards mentioned above contain the following clauses:
- Context of the organization
- Leadership
- Planning
- Support
- Operation
- Performance evaluation
- Improvement
Download ISO 9001:2015, ISO 14001:2015 and ISO 45001:2018 Requirements Comparison in PDF format and use it as reference materials (or in staff/auditors' training).
Check out our Integrated Management System Manual templates, Procedures and Methodical materials.
Labels:
iso 14001 2015,
iso 45001 2018,
iso 9001 2015,
ISO management system standards requirements comparison,
management system requirements comparison
Wednesday, October 4, 2017
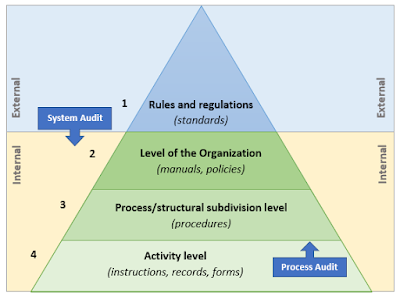
A system audit vs. a process audit
ISO 9001:2015 defines a System as a set of interrelated processes.
While a Process is a set of interrelated or interacting activities, which transforms inputs into outputs.
When auditing, it is important to deeply understand the difference between the two and the moment when a group of processes become a system. Conducting system and process audits at the same time could be confusing and ineffective.
On the document triangle below the first two levels describe the System in a whole, while the two bottom levels describe the process.
System audits start from the top moving down into details, while the process audits start from the bottom of the document pyramid.
To summarize from the auditing perspective:
More information in Which is it – a system or process audit? by J.P.Russel
While a Process is a set of interrelated or interacting activities, which transforms inputs into outputs.
When auditing, it is important to deeply understand the difference between the two and the moment when a group of processes become a system. Conducting system and process audits at the same time could be confusing and ineffective.
On the document triangle below the first two levels describe the System in a whole, while the two bottom levels describe the process.
System audits start from the top moving down into details, while the process audits start from the bottom of the document pyramid.
To summarize from the auditing perspective:
- A system audit is an audit of a system or subsystem against system requirements. (conformity or nonconformity of the system).
- A process audit is an audit of individual processes against predetermined process steps or activities. (inefficiencies and areas for improvement).
More information in Which is it – a system or process audit? by J.P.Russel
| ISO 9001:2015 Products | ISO 14001:2015 Products | ISO 50001:2011 Products |
| IMS Products | ||
Labels:
9001 2015 audit,
first party audits,
quality audits
Friday, September 29, 2017
QMS Questions answered: Audit frequencies
Within the QMS system the core business processes and the supporting 9001 standard requirements procedures/processes have been identified and separated with the specific intent of auditing them at different intervals within the 3-year cycle.
For example, the core business processes will be audited either weekly/monthly/quarterly/bi-annually/annually and the supporting 9001 standard requirements procedures/processes will be audited once within the 3-year cycle because the risk of change is considered low.
Should the auditing of the standard requirements take place each year within a 3-year cycle?
The periodicity of internal audits is not regulated by the requirements of ISO 9001:2015.
Therefore, any audit cycle is acceptable. However, the frequency greater than 1 year may cause difficulties in conducting annual supervisory audits to prove that the QMS is maintained.
A good and most common practice is to audit every process once a year.
For example, the core business processes will be audited either weekly/monthly/quarterly/bi-annually/annually and the supporting 9001 standard requirements procedures/processes will be audited once within the 3-year cycle because the risk of change is considered low.
Should the auditing of the standard requirements take place each year within a 3-year cycle?
The periodicity of internal audits is not regulated by the requirements of ISO 9001:2015.
Therefore, any audit cycle is acceptable. However, the frequency greater than 1 year may cause difficulties in conducting annual supervisory audits to prove that the QMS is maintained.
A good and most common practice is to audit every process once a year.
| ISO 9001:2015 Products | ISO 14001:2015 Products | ISO 50001:2011 Products |
| IMS Products | ||
Monday, September 11, 2017
Nonconformity Management
What are the major changes to the nonconformity management procedure according to the new version of ISO 9001:2015 (vs. ISO 9001:2008)?
The answers to this question can be found in ISO 9001:2015 Transition Gap Analysis Transition Guidelines.
Nonconforming process outputs, products or services are the object of control (ISO 9001:2008 defined nonconforming products as an object of control).
Thus, the requirements where extended:
‘The organization shall deal with nonconforming outputs in one or more of the following ways:
a) correction (vs. ‘to eliminate the detected nonconformity’ in ISO 9001:2008 cl.8.3. a);
b) segregation, containment, return or suspension of provision of products and services;
c) informing the customer;
d) obtaining authorization for acceptance under concession: (In ISO 9001:2008 this was worded as follows: ‘by authorizing its use, release or acceptance under concession by a relevant authority and, where applicable, by the customer’ (cl.8.3. b)).
Conformity to the requirements shall be verified when nonconforming outputs are corrected.’
ISO 9001:2015 Cl.8.7.1
ISO 9001:2015 provides more details regarding retaining of documented information (records) that:
‘a) describes the nonconformity;
b) describes the actions taken;
c) describes any concessions obtained;
d) identifies the authority deciding the action in respect of the nonconformity.’
Purchase ISO 9001:2015 Transition Gap Analysis Transition Guidelines - over 50% off in September/October 2017.
The answers to this question can be found in ISO 9001:2015 Transition Gap Analysis Transition Guidelines.
Nonconforming process outputs, products or services are the object of control (ISO 9001:2008 defined nonconforming products as an object of control).
Thus, the requirements where extended:
‘The organization shall deal with nonconforming outputs in one or more of the following ways:
a) correction (vs. ‘to eliminate the detected nonconformity’ in ISO 9001:2008 cl.8.3. a);
b) segregation, containment, return or suspension of provision of products and services;
c) informing the customer;
d) obtaining authorization for acceptance under concession: (In ISO 9001:2008 this was worded as follows: ‘by authorizing its use, release or acceptance under concession by a relevant authority and, where applicable, by the customer’ (cl.8.3. b)).
Conformity to the requirements shall be verified when nonconforming outputs are corrected.’
ISO 9001:2015 Cl.8.7.1
ISO 9001:2015 provides more details regarding retaining of documented information (records) that:
‘a) describes the nonconformity;
b) describes the actions taken;
c) describes any concessions obtained;
d) identifies the authority deciding the action in respect of the nonconformity.’
Purchase ISO 9001:2015 Transition Gap Analysis Transition Guidelines - over 50% off in September/October 2017.
Thursday, September 7, 2017
Contract Review Metrics
What factors can be used to measure the effectiveness of the Contract review process?
Some suggest to use a quote-hit ratio to obtain a metric on the percentage of orders obtained from quotes. Not in all companies this would be an effective or accurate method, since many quotes are done verbally or through e-mails.
Are there any other ways to determine effectiveness in this process?
There is a universal and well-proven method for any QMS process effectiveness evaluation – the Expert Evaluation.
Each of the 5-7 experts evaluates the effectiveness, for example, on a 10-point scale. The maximum and minimum ratings are discarded and the arithmetic mean is determined.
The main thing is the awareness of experts, as well as disinterest in obtaining certain result. This ensures objectivity.
To evaluate the effectiveness of Contract analysis, the following criteria can also be used:
Some suggest to use a quote-hit ratio to obtain a metric on the percentage of orders obtained from quotes. Not in all companies this would be an effective or accurate method, since many quotes are done verbally or through e-mails.
Are there any other ways to determine effectiveness in this process?
There is a universal and well-proven method for any QMS process effectiveness evaluation – the Expert Evaluation.
Each of the 5-7 experts evaluates the effectiveness, for example, on a 10-point scale. The maximum and minimum ratings are discarded and the arithmetic mean is determined.
The main thing is the awareness of experts, as well as disinterest in obtaining certain result. This ensures objectivity.
To evaluate the effectiveness of Contract analysis, the following criteria can also be used:
- the time taken to sign the contract;
- the ratio of contracts completed in time to the total number of contracts signed;
- the number of customer complaints (an absolute value or in relation to the total number of contracts) - this criterion is applicable to several processes, including Production and service provision process.
Have a QMS related question? Get it answered by an expert!
Labels:
Contract analysis metrics,
contract review process,
effectiveness evaluation; contract analysis process,
QMS process effectiveness
Wednesday, August 30, 2017
ISO/DIS 45001:2017 OH&S management system manual template
We have added a new product to our Management System Documents and Templates collection: ISO/DIS 45001:2017 OH&S Manual Template.
The proposed OH&S Manual is written in sufficient detail. This allows you to use it to solve the following tasks:
Features
Download the 16-page pdf preview of the ISO/DIS 45001:2017 OH&S Manual Template.
The preview contains the following sections:
The proposed OH&S Manual is written in sufficient detail. This allows you to use it to solve the following tasks:
- OH&S Management system audit and certification.
- Understanding of how the OH&S management system operates by senior leadership and personnel.
- Personnel training. Practice shows that the OH&S Management System Manual is often the only source of knowledge about the operation of the OH&S management system for certain categories of personnel, including senior leadership.
- The organization may find feasible to make the OH&S Management System Manual the only documented procedure for the OH&S management system. The proposed OH&S management system manual allows this.
- The OH&S management system manual can be provided to interested parties to demonstrate the effectiveness of the organization's OH&S management system.

Features
- OH&S Manual Template is based on Plan-Do-Check-Act model
- The following standards are referenced:
- Draft International Standard ISO/DIS 45001.2:2017 Occupational health and safety management systems — Requirements with guidance for use
- International Standard ISO 9000:2015 Quality management systems- Fundamentals and vocabulary
- Document is easy to customize in Microsoft Word - no special software required!
- User-friendly format and professional layout - reviewed and approved by experienced auditors.
- 100 pages MS Word document
- Diagrams and models
- Type of delivery: Instant download
Download the 16-page pdf preview of the ISO/DIS 45001:2017 OH&S Manual Template.
The preview contains the following sections:
- 4.2 Understanding the needs and expectations of workers and other interested parties
- 4.3 Determining the Scope of the OH&S Management System
- 4.4. OH&S Management System
- 5.4 Consultation and participation of workers
For more information about the OH&S manual template and to find out most up to date pricing information - please visit our website at
| ISO 9001:2015 Products | ISO 14001:2015 Products | ISO 50001:2011 Products |
| IMS Products | ||
Friday, August 18, 2017
Clause 6.1 Actions to address risks and opportunities QnA
Question:
Is it possible to have two phase of Risk Based Thinking implementation in ISO 9001:2015?
I noticed that in clause 6.1, they mention the relation between this clause and clause 4.1 and 4.2, which is a new requirement, where organization shall identify the interested parties (either internal and external) and address the risk and opportunity. However, in the same clause of 6.1, they mention that the risk/opportunity addressed must be proportionate to product conformity, which still need us to do risk assessment to manufacturing process of product.
As to summarize, the two phase will be like:
Phase 1: Address Risk/Opportunity for Interested Parties
Phase 2: Risk Assessment towards process.
Answer:
The first paragraph of ISO 9001 Clause 6.1 is talking about a mandatory set of requirements that must be taken into account in any QMS planning, namely:
This should be interpreted in a way that the requirements of clause 6.1 are applied Together with clauses 4.1 and 4.2 in achieving planning objectives.
As for addressing of risks and opportunities, these requirements are applicable to all QMS processes (cl 4.4).
Is it possible to have two phase of Risk Based Thinking implementation in ISO 9001:2015?
I noticed that in clause 6.1, they mention the relation between this clause and clause 4.1 and 4.2, which is a new requirement, where organization shall identify the interested parties (either internal and external) and address the risk and opportunity. However, in the same clause of 6.1, they mention that the risk/opportunity addressed must be proportionate to product conformity, which still need us to do risk assessment to manufacturing process of product.
As to summarize, the two phase will be like:
Phase 1: Address Risk/Opportunity for Interested Parties
Phase 2: Risk Assessment towards process.
Answer:
The first paragraph of ISO 9001 Clause 6.1 is talking about a mandatory set of requirements that must be taken into account in any QMS planning, namely:
- internal and external context of the organization (cl.4.1);
- needs and expectations (balance of interests) of interested parties (cl.4.2);
- identification of risks and opportunities, which are discussed further in clause 6.1.
This should be interpreted in a way that the requirements of clause 6.1 are applied Together with clauses 4.1 and 4.2 in achieving planning objectives.
As for addressing of risks and opportunities, these requirements are applicable to all QMS processes (cl 4.4).
Labels:
QMS QnA,
risk management in iso 9001:2015
Wednesday, August 16, 2017
Why should you prefer an Integrated management system (IMS) or not?
With more ISO standards being drafted on a common framework, it is more straightforward to integrate them.
More people find using IMS very beneficial because it helps avoid redundancy of efforts and makes management system truly agile and effective.
Pros of the IMS:
- Using IMS minimizes the duplication of a number of key clauses (Risks, Communication, Nonconformity and corrective actions, Continual improvement, etc.).
- Simplified documentation helps make better choices and allows almost real-time decisions.
Cons of the IMS:
- Difficult to find truly integrated Auditors. QMS auditors often have little to no training in EMS and Safety. You may need to keep internal audit teams separate or plan on getting them additional training on the basics in OSHA and Environmental systems above and beyond the standards.
Choosing to build an IMS should never be driven by desire for certification.
If the management decides to integrate all or some of the aspects described in the different ISO standards into their vision and objectives, it is their strategic decision.
If the management decides to integrate all or some of the aspects described in the different ISO standards into their vision and objectives, it is their strategic decision.
Integrated Management System products
Friday, July 14, 2017
Format of QMS Documented information: Electronic or Printed?
Should the final draft of the Quality manual and procedures be printed?
What happens if we choose to keep the printed copies?
According to the requirements of ISO 9001:2015 (cl. 7.5.2.b) the Organization chooses the documented information format (electronic or printed(hard) copy).
Is it more difficult to manage a hard copy of the Manual, including making changes.
On the other hand, it is a common practice to have a hard copy of the Manual for familiarization and training purposes. In this case, such a copy is not supposed to be managed, and it will not be updated when changes are made.
What happens if we choose to keep the printed copies?
According to the requirements of ISO 9001:2015 (cl. 7.5.2.b) the Organization chooses the documented information format (electronic or printed(hard) copy).
Is it more difficult to manage a hard copy of the Manual, including making changes.
On the other hand, it is a common practice to have a hard copy of the Manual for familiarization and training purposes. In this case, such a copy is not supposed to be managed, and it will not be updated when changes are made.
| ISO 9001:2015 Products | ISO 14001:2015 Products | ISO 50001:2011 Products |
| IMS Products | ||
Labels:
iso 9001 2015 qms development tips,
ISO 9001 2015 QMS document tips,
ISO 9001 2015 QMS documented information
Wednesday, July 12, 2017
Quality Management Principles
ISO 9001:2015 - the most widely used ISO standard in the world - is based on a number of quality management principles including: strong customer focus, leadership, engagement of people, the process approach, continual improvement, evidence-based decision making, and relationship management.
ISO publication Quality Management Principles gives an overview of the ISO 9001:2015 QMP.
The seven quality management principles are :
- Customer focus
- Leadership
- Engagement of people
- Process approach
- Improvement
- Evidence-based decision making
- Relationship management
Access the full publication Quality Management Principles
A collection of ISO 9001:2015 Documents and Templates
ISO 9001:2015 Quality Manual temporary price reduction!
ISO 9001:2015 Quality System Procedures
Monday, July 10, 2017
Remote co-authoring of QMS documented information
 Can the documents like Manuals and procedures be placed in the cloud where all responsible team members can contribute to it?
Can the documents like Manuals and procedures be placed in the cloud where all responsible team members can contribute to it? How do others handle these kinds of situations?
It is a possible practice to co-author QMS documented information remotely.
We offer a couple of simple tips to smooth the process:
- Assign a Team leader who has the most experience in the QMS development.
- Make changes to the documented information having the ‘Track changes’ option ON.
- Team leader is solely responsible for accepting the changes and finalizing the document.
All QMS documents and templates we carry are suitable for remote co-authoring.
Note: When setting up the co-authoring environment pay close attention to the access configuration and information security of the solution.
| ISO 9001:2015 Products | ISO 14001:2015 Products | ISO 50001:2011 Products |
| IMS Products | ||
Labels:
iso 9001 2015 qms development tips,
ISO 9001 2015 QMS document tips,
ISO 9001 2015 QMS documented information
Thursday, June 29, 2017
Integrated system (IMS) vs. Quality and Environment separately
Some of the benefits of having an Integrated system (IMS) vs. Quality and Environment separately include
Manual gives detailed description of the IMS processes and their interaction. (Click to download a sample IMS processes interaction diagram)
The depth of the description is enough for the Organization to decide that this IMS Manual is the only IMS document, which summarizes all mandatory Procedures as well as the procedures that regulate processes in accordance with the existing good practices.
This could be a rational decision of the Organization that provides services for small and mid-size manufacturing companies.
See the IMS section on our website for information about the template.
- IMS is easier to control because Organization’s management system is essentially a ONE system. It could comply with several Standards and in this sense, is called integrated
- Duplication of processes is eliminated.
- Documented information volume is decreased and thus its management is simplified
- Certification, oversight and internal audits are significantly simplified as a result of the possibility of conducting a combined audit (simultaneous audit of several systems).
- This all above results into saving recourses on maintaining the Integrated system in operating condition.
Manual gives detailed description of the IMS processes and their interaction. (Click to download a sample IMS processes interaction diagram)
The depth of the description is enough for the Organization to decide that this IMS Manual is the only IMS document, which summarizes all mandatory Procedures as well as the procedures that regulate processes in accordance with the existing good practices.
This could be a rational decision of the Organization that provides services for small and mid-size manufacturing companies.
See the IMS section on our website for information about the template.
| ISO 9001:2015 Products | ISO 14001:2015 Products | ISO 50001:2011 Products |
Friday, June 2, 2017
Tips for staff undergoing an ISO 9001:2015 audit
What tips do you provide your front-line staff who are going to be interviewed for either an internal or external ISO 9001:2015 audit?
If the personnel already has experience of passing the ISO 9001:2008 certification audit, give them the following advice:
1. Employees should prepare the answers to the following auditor’s questions:
1.1. Risk based thinking (cl.4.4.1.f, cl.6.1):
1.2. Organizational knowledge (cl.7.1.6):
2. Managers should prepare the answers to the following auditors questions:
2.1 Context of the Organization (cl.4.1):
2.2. Understanding the needs and expectations of interested parties (cl.4.2):
3. Production personnel should prepare answers to such questions of auditors (cl.8.5.1.g):
If the personnel already has experience of passing the ISO 9001:2008 certification audit, give them the following advice:
1. Employees should prepare the answers to the following auditor’s questions:
1.1. Risk based thinking (cl.4.4.1.f, cl.6.1):
- Demonstrate examples of risk assessment (identification, analysis, evaluation) in the activities that you are performing (in the Process).
- Demonstrate examples of risk treatment.
- What projects to improve QMS (to enhance desirable effects or to prevent undesired effects) were initiated as a result of Risk treatment.
1.2. Organizational knowledge (cl.7.1.6):
- How do you access the knowledge of the Organization?
- Demonstrate examples of using the Organization's knowledge in the activities that you are doing (in the Process).
2. Managers should prepare the answers to the following auditors questions:
2.1 Context of the Organization (cl.4.1):
- Where do you get information about the external and internal context of the organization?
- Provide examples of use of information about the external and internal context of the organization when making decisions.
2.2. Understanding the needs and expectations of interested parties (cl.4.2):
- Who are the interested parties?
- Give examples of considering the requirements of interested parties in your activities.
3. Production personnel should prepare answers to such questions of auditors (cl.8.5.1.g):
- Give an example of human error prevention activities in your processes.
Thursday, May 18, 2017
Customer communication - contingency actions requirements
IS0 9001:2015
Clause 8.2.1 Customer communication
Communication with customers shall include: • e) establishing specific requirements for contingency actions, when relevant. |
How do you establish SPECIFIC requirements with regard to CONTINGENCIES?
One of the options for meeting this requirement is inclusion of ‘Force Majeure Actions’ section in the consumer contracts.
An example of such a section can have the following form:
In the event of force majeure, neither party shall be liable for failure to fulfill its obligations or delay in performance under the Contract.
Force majeure circumstances are:
- a natural disaster,
- a strike at one of the parties’ organization,
- government body’s restrictions,
- as well as other circumstances of force majeure that make the performance of obligations of one of the parties impossible.
In case such Party does not notify another Party within the period specified above, it shall be deprived of the right to refer to these circumstances in the future. Chamber of Commerce certificate is the Evidence of validity of force majeure.
ISO 9001:2015 Quality System Procedure Contract Analysis
Complete set of ISO 9001:2015 quality system procedures on sale now!
Friday, May 5, 2017
Environmental Targets
What sort of EMS targets could be set, when preparing for ISO 14001 certification when the Organization has very little environmental impact? (i.e. - A small office with very little environmental impact
The traditional wording of environmental targets for the office are:
1. Reduce the power consumption by at least 6%.
2. Reduce the heat consumption by at least 8%.
3. Reduce consumption of consumables related to office activities:
3.1. Paper - no less than 12%.
3.2. Printer cartridges - no less than 20%.
4. Organize separate disposal of drinking cups, wrapping paper and pizza boxes after lunch.
The traditional wording of environmental targets for the office are:
1. Reduce the power consumption by at least 6%.
2. Reduce the heat consumption by at least 8%.
3. Reduce consumption of consumables related to office activities:
3.1. Paper - no less than 12%.
3.2. Printer cartridges - no less than 20%.
4. Organize separate disposal of drinking cups, wrapping paper and pizza boxes after lunch.
| ISO 9001:2015 Products | ISO 14001:2015 Products | ISO 50001:2011 Products |
Friday, April 28, 2017
ISO 9001: Management of Quality or the Quality Management
Which does ISO 9001 emphasize more, the Management of Quality or the Quality of Management?
The majority of newbies who just become familiarizing themselves with ISO 9001 international standard ask the same question:
‘ISO 9001 – is a management system of what?’
Taking into account the following:
- the QMS requirements cover almost all elements of the enterprise management system;
- the requirements are worded based on analysis and synthesis of best international practices suitable for all;
- ISO 9001 is the core of the entire family of management systems standards;
So the following formula is valid: 'QMS = Quality of Management = Quality Business'
 |
| On Sale now! |
Check our ISO 9001:2015 Documents and Templates collection
A complete set of ISO 9001:2015 quality system procedures
Tuesday, April 18, 2017
Risk Management for QMS and EMS
Can we merge Risk Management for QMS and EMS?
If yes what is the common criteria?
Integration of Risk Management for QMS and EMS is highly advisable.
A unified methodology does not confuse employees, makes the organization's management system more "slender", etc.International standards ISO 31000: 2009 and ISO 31010: 2009 are the common criteria.
There are two possible approaches:
- In full - risk management system is developed and implemented in accordance with ISO 31000:2009, as part of the organization's management system. A simple tried-and-tested option is to word Risk Management Manual in a single documented procedure.
- At minimum - risk management methodology is developed that is uniform for all systems. A set of methods suitable for the organization's tasks should be taken from ISO 31010:2009. An example of a simple risk management technique can be found in ISO 9001:2015 Actions to Address Risks and Opportunities Methodical Manual.
Examples of risk management description in the system manuals can be found:
Labels:
ems risk management,
EMS risk management criteria,
iso 14001 risk management,
QMS EMS integration,
qms risk based thinking
Monday, April 10, 2017
ISO 14001:2015 EMS Manual Template
CBG inc. launches new product - ISO 14001:2015 EMS Manual Template.
The proposed EMS Manual is written in sufficient detail. This allows you to use it to solve the following tasks:
Check our website for the detailed description of the EMS Manual Template or Download the preview of the template
The proposed EMS Manual is written in sufficient detail. This allows you to use it to solve the following tasks:
- EMS Audits and certification.
- Understanding EMS operation by senior management. Practice shows that EMS Manual often is the only source of knowledge about the EMS operation for certain categories of personnel, including senior management.
- The organization may find it feasible to make the EMS Manual the only documented procedure. The proposed EMS Manual allows this.
- The EMS manual can be provided to interested parties, including state environmental supervisory bodies, residents of the region, etc. to prove the effectiveness of the EMS of the organization.
- Personnel training.
Check our website for the detailed description of the EMS Manual Template or Download the preview of the template
Labels:
iso 14001 2015 ems manual template,
iso 14001 2015 environmental management system,
iso 14001 ems certification,
iso 14001 environmental management
Thursday, April 6, 2017
Quality Objectives
QMS objectives will vary from Organization to Organization but are some of the more common objectives professionals see?
Do they scores on customer feedback surveys, lack of complaints measured over a financial year, etc.?
The top notch practice when wording the quality objectives – is using two methodologies:
1) QFD Analysis
Good results could be achieved from:
This method is the basis for all standard management systems implementation.
We word some global objectives. The activities for them become the objectives for lower levels, etc.
In ISO 9001:2015 requirements, the chain is as follows:
Unfortunately, in practice, this is rarely used.
ISO 9001:2015 Quality Manual Template
Collection of ISO 9001:2015 documents, templates, and procedures
Do they scores on customer feedback surveys, lack of complaints measured over a financial year, etc.?
The top notch practice when wording the quality objectives – is using two methodologies:
1) QFD Analysis
Good results could be achieved from:
- Converting the ‘customer requirements’ body into a set of "engineering characteristics of products." As a result, we get a list of engineering characteristics of products, ranked according to the degree of satisfaction of customers' requirements. Next, quality goals are set, aimed at improving the engineering characteristics of products, which are on the first positions in the list.
- Transforming ‘product defects’ list into a set of ‘technological operations’. As a result, we get a list of technological operations, ranked according to the degree of influence on the appearance of defects. Further, quality objectives are set, aimed at improving technological operations, where the defects occur most often (the first in the list).
This method is the basis for all standard management systems implementation.
We word some global objectives. The activities for them become the objectives for lower levels, etc.
In ISO 9001:2015 requirements, the chain is as follows:
Unfortunately, in practice, this is rarely used.
ISO 9001:2015 Quality Manual Template
Collection of ISO 9001:2015 documents, templates, and procedures
Labels:
customer feedback,
customer requirements,
product defects,
qfd analysis,
quality goals of the organization,
quality objectives,
quality policy
Monday, April 3, 2017
Organizational structure in ISO 9001:2015 Quality manual
Does ISO 9001:2015 require Quality Manual to contain brief information of the Organization?
The structure of documented information as per ISO 9001: 2015 (cl 7.5.1) does not contain requirements to have a Quality Manual, as was the case with the ISO 9001: 2008 documentation structure.
Thus, Quality manual is not a mandatory QMS document.
The existence of Quality Manual is a decision of the organization in accordance with good practices.
This is attributable to:
If the organization makes a decision to have a Quality Manual, then:
It is a good practice to also add elements of social responsibility, corporate traditions, etc to section 7.1.4.
Check out our collection of ISO 9001:2015 documents and templates
The structure of documented information as per ISO 9001: 2015 (cl 7.5.1) does not contain requirements to have a Quality Manual, as was the case with the ISO 9001: 2008 documentation structure.
Thus, Quality manual is not a mandatory QMS document.
The existence of Quality Manual is a decision of the organization in accordance with good practices.
This is attributable to:
- Feasibility of QMS operation description in general and linking the processes to solve organization's management tasks;
- Training objectives;
- Audit objectives;
- Acquaintance of interested parties, including customers, with the QMS.
If the organization makes a decision to have a Quality Manual, then:
- The organizational structure should be given in section 5.3 of the document.
- It is advisable to provide brief information about the organization to the auditors and interested parties. This can be done in section 7.1.4.
It is a good practice to also add elements of social responsibility, corporate traditions, etc to section 7.1.4.
Check out our collection of ISO 9001:2015 documents and templates
Labels:
iso 9001 2015 good practices,
iso 9001 2015 organizational structure,
iso 9001 2015 qms implementation tips,
iso 9001 2015 quality manual
Tuesday, March 21, 2017
Auditing for control and improvement
When developing and implementing a QMS, quality managers often ask themselves these questions:
- Is this procedure mandatory?
- Do I need to maintain those records?
- What are the documents required by ISO 9001?
It is manager's job to establish and implement controls and ensure there is continual improvement. In the absence of specific guidance in performance standards (required procedures, records, or schedules) it is essential that management be able to demonstrate conformance to requirements.
It is especially important when preparing for the audit (both internal and external).
It is a good practice to maintain Quality Manual and Quality Standard procedures as documented information.
More on Auditing for control and improvement - in the corresponding article by J.P.Russel
ISO 9001:2015 Documents and Templates collection
Friday, March 10, 2017
Implementing 14001 within existing 9001
When implementing 14001 within existing 9001 should both systems - QMS and EMS be implemented as separate ones with separate documentation, or may EMS be included as one of QMS processes?
For example, may I have one internal audit procedure, internal audit plan and carried out integrated internal audit for both QMS AND EMS?
Have one Management Review for both systems?
Can I just build one system describing both standards?
What is the common practice for integrated systems – how do you do it?
(We are planning to implement 27 001 and 20 000 eventually as well…)
Integration of standard management systems, including 9001 and 14001 is not only possible, but also necessary!
The most recent versions of Management Standards have identical structure namely for the integration purposes.
All these makes the integration fairly simple.
While integrating, ‘including EMS as one of QMS processes’ is not the best approach.
It is better to build one system describing both standards.
A good practice is to build integrated systems in two phases:
1) Build an ISO 9001 QMS as a base
2) Build the integrated system using the ‘Fill the gaps’ method
In this case, gaps - are the distinctive sections of the ‘Integrated Management System Manual in compliance with ISO 9001: 2015 and ISO 14001: 2015’ and the expediency of developing additional processes can be summarized in the table:
Further, ISO 27 001, 20 000 systems could be added on.
ISO 9001:2015 Quality Manual Template
ISO 9001:2015 Documents and Templates Collection
For example, may I have one internal audit procedure, internal audit plan and carried out integrated internal audit for both QMS AND EMS?
Have one Management Review for both systems?
Can I just build one system describing both standards?
What is the common practice for integrated systems – how do you do it?
(We are planning to implement 27 001 and 20 000 eventually as well…)
Integration of standard management systems, including 9001 and 14001 is not only possible, but also necessary!
The most recent versions of Management Standards have identical structure namely for the integration purposes.
All these makes the integration fairly simple.
While integrating, ‘including EMS as one of QMS processes’ is not the best approach.
It is better to build one system describing both standards.
A good practice is to build integrated systems in two phases:
1) Build an ISO 9001 QMS as a base
2) Build the integrated system using the ‘Fill the gaps’ method
Further, ISO 27 001, 20 000 systems could be added on.
ISO 9001:2015 Quality Manual Template
ISO 9001:2015 Documents and Templates Collection
Labels:
14001 processes,
fill the gap,
implement 14001 within exisitng 9001,
iso 9001 14001 integration
Thursday, March 9, 2017
Digital Transformation in Quality Management
Emerging digital technologies, increasing competition and changing customer demands put extra pressure on the businesses. In order to survive in the digital age, companies have to adapt to the changes and modify their operations through digital transformation.
While adapting new elements of digitization, the organization should bear in mind that that in order to achieve financial success, the investment into the digital transformation has to be combined with the clear vision of the process, careful coordination, engagement, and leadership.
For more digital transformation information, its benefits, potential pitfalls and ways to reach the competitive advantage, refer to The Advantages of Digital Maturity
ISO 9001:2015 does not directly require the organization to undergo digital transformation, though some of ISO 9001:2015 requirements are aligned with it.
MIT Sloan School of Management identifies 9 areas of digital transformation. Let’s review them with regards to the ISO 9001:2015 clauses.
Customer focus
Top management shall demonstrate leadership and commitment with respect to customer focus by ensuring that:
a) customer and applicable statutory and regulatory requirements are determined, understood and consistently met;
b) the risks and opportunities that can affect conformity of products and services and the ability to enhance customer satisfaction are determined and addressed;
c) the focus on enhancing customer satisfaction is maintained
ISO 9001:2015 cl.5.1.2
Digitally transforming Customer Experience is the most visible and beneficial part of the Digital transformation.
1. Customer Understanding. It is a good practice to
Process approach
This International Standard promotes the adoption of a process approach when developing, implementing and improving the effectiveness of a quality management system, to enhance customer satisfaction by meeting customer requirements.
ISO 9001:2014 Cl.0.3.1
Benefits from Transforming Operational Processes are less visible in comparison to transforming the Customer experience and require more investment.
4. Process Digitization. Through automation of production processes, the Organization can improve product quality (avoid human error). The personnel, freed from the repetitive production jobs, can focus on RnD processes.
5. Worker Enablement
6. Performance Management
Digital transformation also Transforms Business Models of the Organizations. They can benefit from pursuing new digital directions of business development or digitally modifying the existing ones.
7. Digitally Modified Businesses
9. Digital Globalization
Digital transformation provides opportunity for the Organization to improve quality of product/services through the way individuals work and collaborate, the way business processes are executed and in the way a company understands and serves customers.
ISO 9001:2015 Documents and Templates collection.
While adapting new elements of digitization, the organization should bear in mind that that in order to achieve financial success, the investment into the digital transformation has to be combined with the clear vision of the process, careful coordination, engagement, and leadership.
For more digital transformation information, its benefits, potential pitfalls and ways to reach the competitive advantage, refer to The Advantages of Digital Maturity
ISO 9001:2015 does not directly require the organization to undergo digital transformation, though some of ISO 9001:2015 requirements are aligned with it.
MIT Sloan School of Management identifies 9 areas of digital transformation. Let’s review them with regards to the ISO 9001:2015 clauses.
Customer focus
Top management shall demonstrate leadership and commitment with respect to customer focus by ensuring that:
a) customer and applicable statutory and regulatory requirements are determined, understood and consistently met;
b) the risks and opportunities that can affect conformity of products and services and the ability to enhance customer satisfaction are determined and addressed;
c) the focus on enhancing customer satisfaction is maintained
ISO 9001:2015 cl.5.1.2
Digitally transforming Customer Experience is the most visible and beneficial part of the Digital transformation.
1. Customer Understanding. It is a good practice to
- Leverage social media to understand the roots of customer dissatisfaction
- Build online communities to promote brands
- Use Analytic's capability to improve portfolios
- Enhance sales with use of digital presentations and customer Analytic's data
Process approach
This International Standard promotes the adoption of a process approach when developing, implementing and improving the effectiveness of a quality management system, to enhance customer satisfaction by meeting customer requirements.
ISO 9001:2014 Cl.0.3.1
Benefits from Transforming Operational Processes are less visible in comparison to transforming the Customer experience and require more investment.
4. Process Digitization. Through automation of production processes, the Organization can improve product quality (avoid human error). The personnel, freed from the repetitive production jobs, can focus on RnD processes.
5. Worker Enablement
- Remote/virtual collaboration; ability to work from anywhere
- Creating organization wide knowledge banks (7.1.6 Organizational knowledge)
6. Performance Management
- The detailed information gathered from the transaction processing system allow management decision s to be made on the real data – versus the assumptions.
- Virtual collaboration enables better input into the processes, that will lead to the better risk and opportunities assessment
Digital transformation also Transforms Business Models of the Organizations. They can benefit from pursuing new digital directions of business development or digitally modifying the existing ones.
7. Digitally Modified Businesses
- Outsourcing of customer service and post-delivery activity
9. Digital Globalization
- Global shared services promote efficiency and reduce risk.
Digital transformation provides opportunity for the Organization to improve quality of product/services through the way individuals work and collaborate, the way business processes are executed and in the way a company understands and serves customers.
ISO 9001:2015 Documents and Templates collection.
Labels:
digital transformation organization,
iso 9001 digital transformation,
quality and digital transformation
Saturday, March 4, 2017
What did ISO 9001:2015 teach you?
What are the new things - which you ignored about your Quality Management System - did/does the new edition of ISO 9001:2015 teach you?
Our experience in consulting the transition of the organizations to the new version of ISO 9001:2015 showed that the main «new things that were previously ignored» are the following four.
1. System-level analysis of internal and external context of the Organization.
1.1 Gathering information and analysis of the Context components in the following processes: ‘Management review’, ‘Marketing’, ‘Control of personnel’, ‘Design and development or products and (or) services’, ‘Production and service provision’, ‘Control of documented information’.
1.2 Distribution of information about the context internally in the Organization within the framework of control of organizational knowledge and documented information
1.3 Use of information about the context in QMS processes when planning and making management decisions.
The most interesting positive practice was the wording of certain quality objectives, based on the context changes through the application of QFD - matrices.
2. Actions to address risks and opportunities.
On the very first stage the positive outcomes are, at minimum:
3. Analysis of needs and expectations of interested parties (other than consumers and suppliers).
A good practice here is to:
4. Viewing Organizational knowledge as an important resource.
A good practice is to define in the QMS:
You may find it useful to implement all theses in the QSP format.
We have implemented the mentioned above (with the exception of the QFD - matrices) in the
Our experience in consulting the transition of the organizations to the new version of ISO 9001:2015 showed that the main «new things that were previously ignored» are the following four.
1. System-level analysis of internal and external context of the Organization.
1.1 Gathering information and analysis of the Context components in the following processes: ‘Management review’, ‘Marketing’, ‘Control of personnel’, ‘Design and development or products and (or) services’, ‘Production and service provision’, ‘Control of documented information’.
1.2 Distribution of information about the context internally in the Organization within the framework of control of organizational knowledge and documented information
1.3 Use of information about the context in QMS processes when planning and making management decisions.
The most interesting positive practice was the wording of certain quality objectives, based on the context changes through the application of QFD - matrices.
2. Actions to address risks and opportunities.
On the very first stage the positive outcomes are, at minimum:
- Management decisions multi-variance (management started reviewing all possible variants and estimating risks);
- Forward thinking about the consequences (this was not a mass phenomenon earlier).
3. Analysis of needs and expectations of interested parties (other than consumers and suppliers).
A good practice here is to:
- Define processes, where the needs of each interested party is monitored and analyzed, and clearly determine the requirements information format;
- Create the shareholders interests balance table (shareholder’s interest – Organization’s interest), at least in a simplified format.
4. Viewing Organizational knowledge as an important resource.
A good practice is to define in the QMS:
- The nature of knowledge;
- Information media;
- Processes- suppliers of the information – for each knowledge category;
- Level of employees’ access;
- Responsible for information updates.
You may find it useful to implement all theses in the QSP format.
We have implemented the mentioned above (with the exception of the QFD - matrices) in the
Labels:
iso 9001 20015 new things,
iso 9001 2015 good practices,
iso 9001 2015 risk based thinking,
iso 9001 2015 risk management,
iso 9001 2015 transition tips,
iso 9001 lessons learned
Thursday, February 16, 2017
Transitioning from ISO 9001:2008 to ISO 9001:2015: Documentation
Are there any key points to remember in transitioning from ISO 9001:2008 to ISO 9001:2015 especially when it comes to documentation?
For the key points to remember in transitioning from ISO 9001:2008 to ISO 9001:2015 refer to the following sections
4.1. In the section 4.1 of Quality Manual, describe activities to monitor, analyze and communicate the information about the internal and external context including three components
6.1. Actions to address risks and opportunities. Develop and implement a risk management system considering (or even in compliance with) ISO 31000. Such system fits in well with the requirements of ISO 9001:2015. An example of this decision in a concise form, when risk management activation are described in the form of a quality procedure can be found in QSP 6.1-01 Actions to address risks and opportunities.
Other approaches to addressing risks and opportunities and more details on the subject find in Actions to address risks and opportunities: Planning.
6.2. It is necessary to plan achieving the quality objectives (not only define and monitor their implementation as required by ISO 9001:2008).
A good and practically proven methodology is to word quality objectives of the organization in the form of the Organization’s Annual Development Program.
Further details of quality objectives planning are given in the 2nd edition of ISO 9001:2015 Quality Manual.
7.1.5. Monitoring and measuring resources – is aimed at definition of requirements, ensuring availability and serviceability of the three components of resources for monitoring and measuring:
7.1.6. Consider knowledge as an importance resource to operate processes and ensure products and services compliance. Control of organizational knowledge is aimed at:
8.5. While providing and describing the controlled production conditions ensure the implementation of actions to prevent human error.
For the key points to remember in transitioning from ISO 9001:2008 to ISO 9001:2015 refer to the following sections
4.1. In the section 4.1 of Quality Manual, describe activities to monitor, analyze and communicate the information about the internal and external context including three components
- gathering of information and analysis of the components of internal and external context in QMS processes;
- communication of information about the context inside the Organization;
- use of information about the context of the organization.
- defining interested parties relevant to the QMS;
- monitoring and analysis of interested parties’ needs and expectations.
6.1. Actions to address risks and opportunities. Develop and implement a risk management system considering (or even in compliance with) ISO 31000. Such system fits in well with the requirements of ISO 9001:2015. An example of this decision in a concise form, when risk management activation are described in the form of a quality procedure can be found in QSP 6.1-01 Actions to address risks and opportunities.
Other approaches to addressing risks and opportunities and more details on the subject find in Actions to address risks and opportunities: Planning.
6.2. It is necessary to plan achieving the quality objectives (not only define and monitor their implementation as required by ISO 9001:2008).
A good and practically proven methodology is to word quality objectives of the organization in the form of the Organization’s Annual Development Program.
Further details of quality objectives planning are given in the 2nd edition of ISO 9001:2015 Quality Manual.
7.1.5. Monitoring and measuring resources – is aimed at definition of requirements, ensuring availability and serviceability of the three components of resources for monitoring and measuring:
- Measuring equipment (it was in the ISO 9001:2008 requirements);
- Measuring and monitoring methodologies (new);
- Competence of personnel conducting monitoring and measuring (new).
7.1.6. Consider knowledge as an importance resource to operate processes and ensure products and services compliance. Control of organizational knowledge is aimed at:
- Definition of knowledge necessary to operate the processes and achieve products and services compliance;
- Obtaining and maintaining of knowledge
- Ensuring the availability of knowledge to the extent necessary
- Viewing the current knowledge and obtaining access to any necessary additional knowledge and the required updates in response to changing needs and trends.
8.5. While providing and describing the controlled production conditions ensure the implementation of actions to prevent human error.
Labels:
control of documented information,
documenting qms transition to the new version of iso 9001,
qms transition tips
Sunday, February 12, 2017
Product updates: ISO 9001:2015 Quality Manual Template
We have incorporated 12 months’ experience of SO 9001:2015 compliant QMS operation, took into consideration the ISO 9002:2016 requirements and offer the 2nd edition of Quality Manual template.
The new Quality manual template is the detailed description of the QMS processes and their interaction.
The depth of the description is enough for the Organization to decide that this Quality Manual is the only QMS document, which summarizes all mandatory Procedures as well as the procedures that regulate processes in accordance with the existing good practices.
This could be a rational decision of the Organization that provides services for small and mid-size manufacturing companies.
ISO 9001:2015 Quality Manual template package includes:
Quality Manual Template 1st edition
Please note that Quality Manuals’ sections are inter-changeable.
Having access to both editions, you have an opportunity to create your own unique Quality Manual, compile it using sections that are the most suitable for your organization.
ISO 9001:2015 Documents and Templates collection
The new Quality manual template is the detailed description of the QMS processes and their interaction.
The depth of the description is enough for the Organization to decide that this Quality Manual is the only QMS document, which summarizes all mandatory Procedures as well as the procedures that regulate processes in accordance with the existing good practices.
This could be a rational decision of the Organization that provides services for small and mid-size manufacturing companies.
ISO 9001:2015 Quality Manual template package includes:
Quality Manual Template 1st edition
- 54 pages MS Word document (Preview)
- Diagrams and models
- 108 pages MS Word document (Preview)
- Diagrams and models
Please note that Quality Manuals’ sections are inter-changeable.
Having access to both editions, you have an opportunity to create your own unique Quality Manual, compile it using sections that are the most suitable for your organization.
ISO 9001:2015 Documents and Templates collection
Labels:
9001 quality manual template updated,
iso 9001 2015,
iso 9002 2016,
mandatory qms documents,
only QMS document
Thursday, February 9, 2017
Quality Objectives and Planning to achieve them
One of the requirements of the new version of ISO 9001 Standard is that there should be a plan to achieve quality objectives.
How would/have you come up with a plan for achieving quality objectives and how would/have you document(ed) them?
A good and practically proven practice is too word quality objectives of the organization in the form of the Organization’s Annual Development Program, which could have the following structure:
Quality objectives of the Process (or structural subdivision) are set by the process owner (or structural subdivision manager), based on the analysis of two components:
Further details of quality objectives planning are given in the 2nd edition of ISO 9001:2015 Quality Manual.
How would/have you come up with a plan for achieving quality objectives and how would/have you document(ed) them?
A good and practically proven practice is too word quality objectives of the organization in the form of the Organization’s Annual Development Program, which could have the following structure:
- #
- Quality objectives wording
- Process name
- Responsible for the implementation
- Deadlines
- Resources
- Report type
Quality objectives of the Process (or structural subdivision) are set by the process owner (or structural subdivision manager), based on the analysis of two components:
- Tasks resulting from Organization’s Development Program for the year;
- Tasks aimed at the process (or subdivision) development, using own resources.
Further details of quality objectives planning are given in the 2nd edition of ISO 9001:2015 Quality Manual.
Friday, February 3, 2017
Numbering System for ISO documentation based on ISO 9001:2015
As far as I know ISO 9001:2015 do not mandates a specific numbering system for control documents.
Is it true that the organization is left open to adopt one that suits it's organizational needs?
Also I am interested to know the best practices for the numbering system.
Yes, that's right - ISO 9001:2015 does not mandate a specific numbering system for control of documents.
Here is an example of the documented information number structure that has been proven in practice:
1. Abbreviation that identifies the document type in accordance with the Documented information structure adopted by the Organization.
For example:
Example: 7.1- , a group of documents relevant to resources.
3. Index number of the document in the group.
Example: -003 means this is a document #3 in the group.
4. Sometimes it is relevant to give the document’s year of approval in the last part of the id number.
Example: -2017.
QSP 7.5-01-2017 Control of Documented Information
You can find more examples of the QMS documented information numbering on our website in the ISO 9001:2015 Quality System Procedures section
Is it true that the organization is left open to adopt one that suits it's organizational needs?
Also I am interested to know the best practices for the numbering system.
Yes, that's right - ISO 9001:2015 does not mandate a specific numbering system for control of documents.
Here is an example of the documented information number structure that has been proven in practice:
1. Abbreviation that identifies the document type in accordance with the Documented information structure adopted by the Organization.
For example:
- QSP = Quality System Procedure.
- QSF = Quality System Form
Example: 7.1- , a group of documents relevant to resources.
3. Index number of the document in the group.
Example: -003 means this is a document #3 in the group.
4. Sometimes it is relevant to give the document’s year of approval in the last part of the id number.
Example: -2017.
QSP 7.5-01-2017 Control of Documented Information
You can find more examples of the QMS documented information numbering on our website in the ISO 9001:2015 Quality System Procedures section
Labels:
control of documented information best practices,
QMS documented information,
qms questions and answers
Subscribe to:
Comments (Atom)